Colombian Cosmetic Grade CBD and CBG

Colombian Pharmaceutical grade Cannabinoids (CBD, THC, CBG) and Standarized Extracts

Colombian federally registered hemp and cannabis seeds

Low cost, Organic and Sustainable Cultivation
Ranked highest amongst global cannabis companies in the SAM Corporate Sustainability Index issued by S&P Global.
Active Pharmaceutical Ingredients
Aureus offers Colombian and US sourced cannabinoid API derived from Certified hemp and cannabis plants compliant with EU and US pharmacopeia standards.
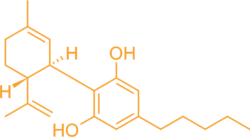
CBD
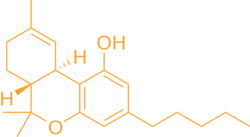
THC
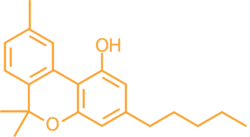
CBN
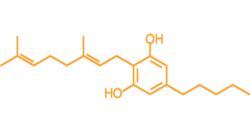
CBG
The Aureus Advantage
In Collaboration With Leading
Academic Institutions
The Avicanna team of expert breeders, cultivators and scientists work in collaboration with plant geneticists and plant scientists from both the University of Guelph in Canada and University of Buenos Aires.



Cannabis Cultivars
A diverse range of THC dominant cultivars that are suitable for different environments and cultivation practices. Cannabinoid expression ranges from THC dominant, to diverse expression of rare cannabinoids and balance THC-CBD offerings. Select cultivars are offered in feminized and standardized seeds but all cultivars are available in clones.
Hemp Cultivars
A diverse range of THC dominant cultivars that are suitable for different environments and cultivation practices. Cannabinoid expression ranges from THC dominant, to diverse expression of rare cannabinoids and balance THC-CBD offerings. Select cultivars are offered in feminized and standardized seeds but all cultivars are available in clones.
As Seen In
Do you want to Become a Wholesaler?
Aureus Value Chain
Aureus is powered by Avicanna Inc., a Canadian biopharmaceutical company focused on cannabinoid research and development. This means Aureus products are developed by combining hands-on scientific experience, and global pharmaceutical industry standards, quality and purity of the API for supplements, cosmetics, and pharmaceutical products.
All Aureus products are tested by an analytical laboratory that provides GMP-compliant analytical testing services to assess product composition, safety and quality. This ensures the highest possible quality of product for clients focusing on consistent and independent quality controls. These include USP and EU pharmacopeia which are core standards relied upon in Europe and the United States that are used to help assess the quality, strength, identity and purity of the Aureus API.